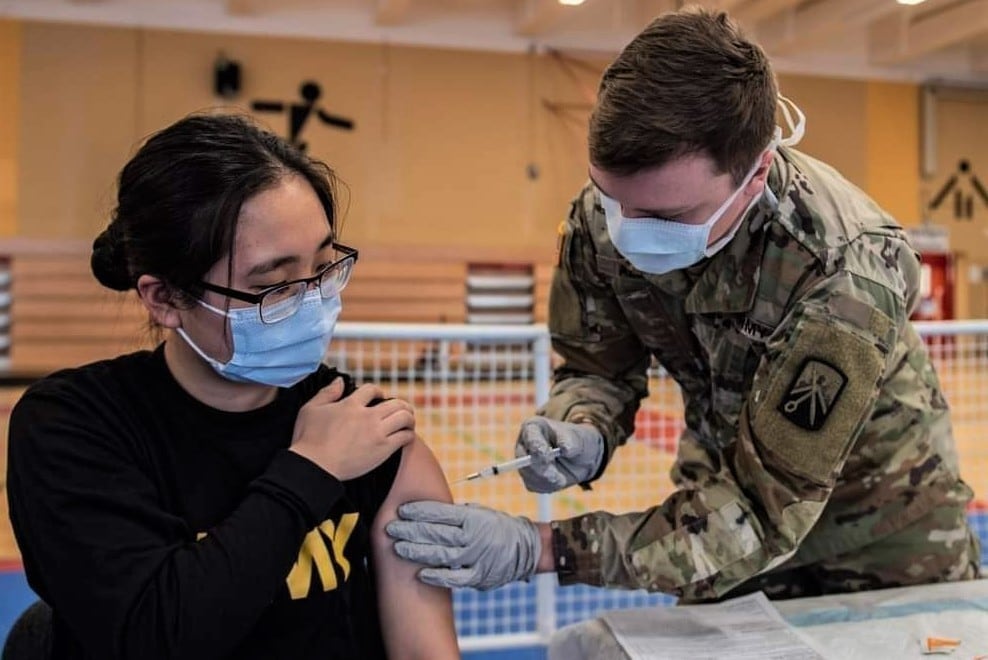
Army officials say they’re not tracking how many soldiers refuse vaccination against the novel coronavirus because the number of troops who want the shot is still greater than the number of doses available.
But the gap is closing, and the Pentagon’s pause on administering the Johnson and Johnson vaccine is expected to lift this week, which will help troops serving in austere locations because the one-shot J&J vaccine is easier to transport and store.
As of Tuesday, about 144,746 U.S. soldiers have been fully vaccinated, according to Pentagon data. Another 136,614 soldiers have been partially vaccinated.
“Everybody needs more vaccines so they can vaccinate their soldiers,” Army Medical Command boss Lt. Gen. R. Scott Dingle said Monday during a call with reporters. “However, we are just getting to that point with the increased production of the vaccine where we’ll be able to meet that demand.”
Vaccination remains optional, and although Army leaders have been lobbying aggressively to get troops comfortable with taking it, the service’s vaccine program leaders say they’re not concerned about refusals right now.
“Until the supply exceeds our demand, we’re really not keeping that much track of it, because at this point, the demand far outstrips supply,” said Brig. Gen. Matthew D. Smith, the Army’s director of operations at the Pentagon.
“Every time a soldier says ‘hey, I’m not interested’ … it basically becomes a next man up drill, next woman up drill, and we just get the shot in the arm of any soldier,” Smith added. “We’re doing that to the tune of about 95 percent of the supplies issued each week.”
That hasn’t stopped the information campaign aimed at encouraging troops to take the vaccine.
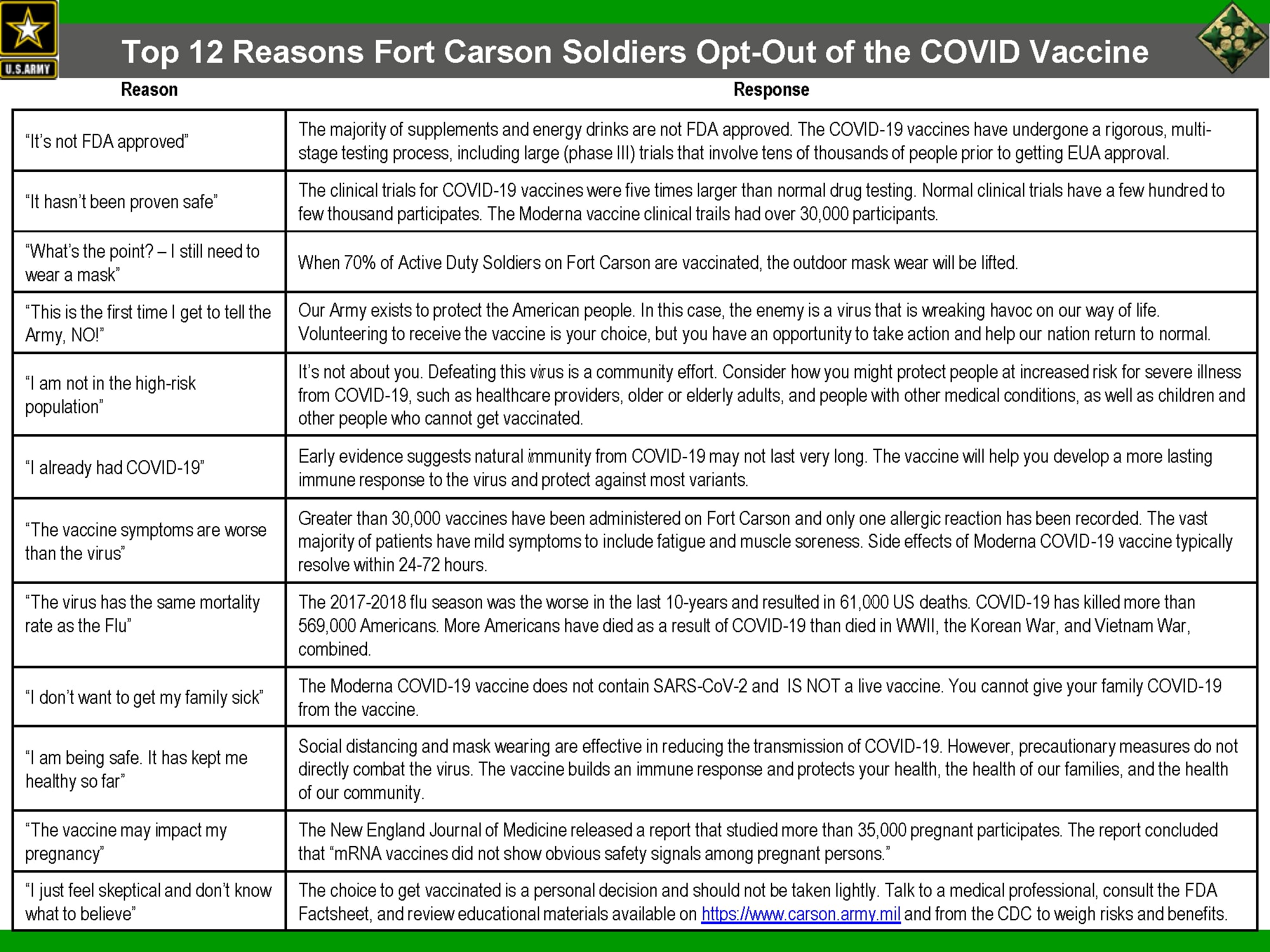
Officials at Fort Carson, Colorado, posted a slide to their Facebook page Monday highlighting what they say are the top 12 reasons soldiers there have been opting out of vaccination.
“It’s not FDA approved,” is one common reason, the slide shows. But the majority of supplements and energy drinks consumed by troops are also not FDA approved, the slides point out.
The COVID-19 vaccines were approved under an emergency use authorization by the FDA, which means they haven’t completed all of the multiple rounds of rigorous testing required before the FDA clears them.
However, the expedited process still requires FDA reviewers to pour over all safety data from the first two phases of clinical trials and partial data from the third phase.
Pentagon leadership can’t make vaccines mandatory until they’re fully cleared by the FDA. And although the president can issue a waiver mandating vaccination, that has not been done and would make little sense, said Dr. Steven Cersovsky, deputy director of the Army Public Health Center, in late February.
“I think the issue now is that we still have a limited supply and we need to prioritize those individuals who receive the vaccine,” Cersovsky said. “We want to stick to that prioritization schema. … And at some point when we have enough vaccines, then I think you’ll see perhaps a decision made as to whether this will be mandatory immunization.”
Kyle Rempfer was an editor and reporter who has covered combat operations, criminal cases, foreign military assistance and training accidents. Before entering journalism, Kyle served in U.S. Air Force Special Tactics and deployed in 2014 to Paktika Province, Afghanistan, and Baghdad, Iraq.