Veterans Affairs officials said this week they’ll continue to use a controversial anti-malarial drug in treatment for coronavirus patients despite medical studies showing significant health risks associated with the medicine.
The decision, relayed to lawmakers by top VA officials, comes as active cases of the fast-spreading illness continue to drop at department hospitals but also as deaths connected from the illness keep rising.
As of Friday morning, 1,110 VA patients had died from coronavirus-related complications in the last three months. That figure is up about 13 percent from one week ago, and more than double the 510 deaths publicly reported by the department on May 1.
RELATED
But VA researchers said the number of active cases at medical centers had dropped to 1,660 by Friday morning, a reduction of about 17 percent in the last week and nearly half the total at the start of the month.
Department officials in recent days have begun plans to partially reopen 20 medical facilities across the country in an effort to return to pre-pandemic operations.
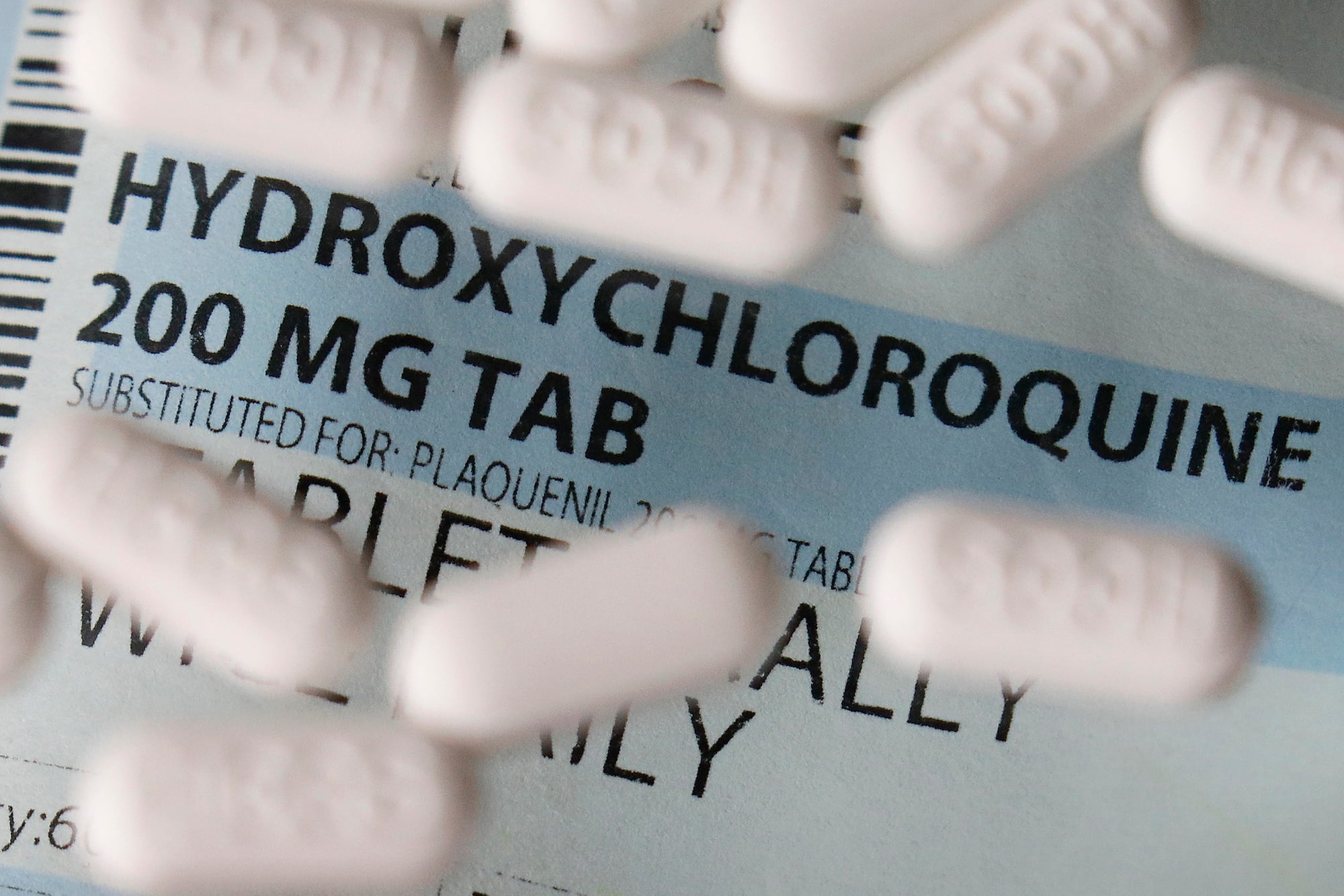
However, lawmakers still have questions about VA’s overall response to the outbreak, including the decision to use hydroxychloroquine as a treatment for some department patients.
The drug has become a source of controversy since President Donald Trump hyped the medicine in early March as a potential cure or vaccine for the virus, despite limited medical testing at the time. Since then several wider studies — including one conducted by VA researchers — have shown serious and potentially fatal side effects associated with its use.
Despite that, Trump recently announced he is taking the drug under a physician’s supervision.
RELATED

In response to congressional questions. VA officials acknowledged that they have treated about 1,300 patients with hydroxychloroquine for coronavirus issues. Department leaders denied any pressure from White House officials to use the drug despite the wider medical concerns.
“The idea that VA health care providers would make treatment decisions based on anything other than the best medical interests of our patients as individuals is preposterous,” officials said in a statement. “VA, like so many medical facilities across this nation, is in a race to keep patients alive during this pandemic, and we are using as many tools as we can.”
But critics dismissed that answer as incomplete and confusing.
“We need to know what the basis was for using this drug against the consensus of science, which called into question its effectiveness in treating COVID-19,” said Senate Minority Leader Chuck Schumer, D-N.Y. “We also need to know who is authorizing these new trials, what facilities are participating and what families are being told.”
RELATED

Department officials said that they will continue to use the drug for coronavirus patients who they deem appropriate for the treatments “in accordance with Food and Drug Administration guidelines.”
On Wednesday, former VA Secretary David Shulkin publicly criticized Trump’s use of hydroxychloroquine, calling it “a drug that has no effectiveness or no known effectiveness, but potential harm.”
VA staff have conducted more than 161,000 coronavirus tests since early March. About 8 percent of all tests have been positive. About 8.5 percent of those positive cases have resulted in a patient’s death.
That fatality rate is well above the 6 percent death rate for cases among all Americans, according to the latest data released by the Centers for Disease Control and Prevention.
However, VA officials have said the mortality data for their patients “cannot be used to compare VA infection or mortality rates with the community because of differences in population risk, test availability, and follow-up.”
Leo covers Congress, Veterans Affairs and the White House for Military Times. He has covered Washington, D.C. since 2004, focusing on military personnel and veterans policies. His work has earned numerous honors, including a 2009 Polk award, a 2010 National Headliner Award, the IAVA Leadership in Journalism award and the VFW News Media award.





